In a groundbreaking move, the U.S. Food and Drug Administration (FDA) has approved a new type of pain medication, Journavx, marking the first new class of painkillers in over two decades. This approval signals a potential game-changer in the fight against opioid addiction, offering an alternative to traditional pain management without the risk of dependence.
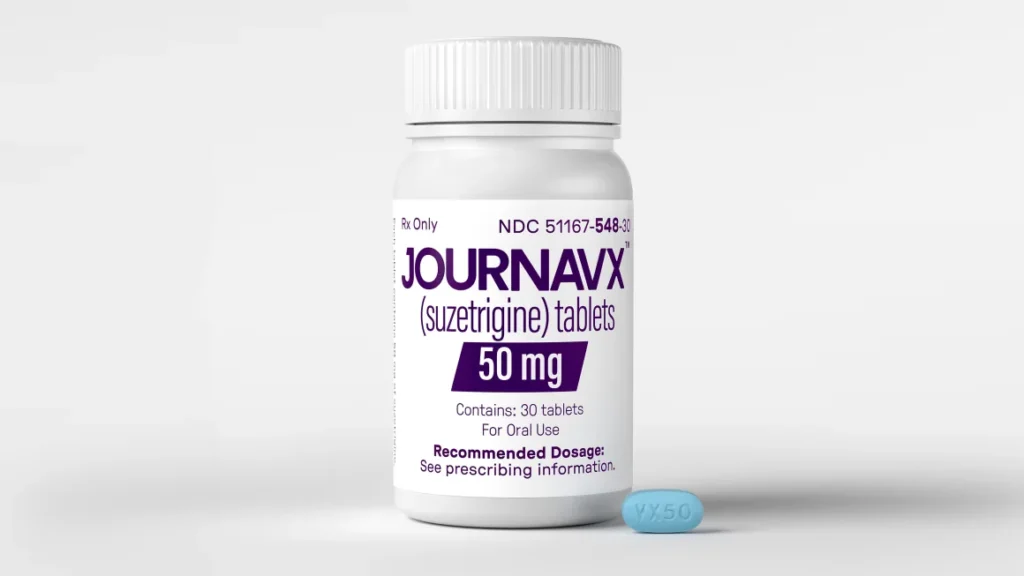
Suzetrigine, sold as Journavx, works by blocking specific nerve channels involved in pain signaling, reducing discomfort without activating the brain’s reward centers where addiction can develop. In clinical trials, the drug showed effectiveness similar to opioids combined with acetaminophen but with fewer side effects like itching, muscle spasms, and rashes.
However, despite its promising benefits, Journavx comes with a price tag that’s considerably higher than traditional painkillers, raising questions about accessibility. Will this breakthrough pave the way for a safer, non-addictive future in pain management—or will its cost put it out of reach for many who need it most?